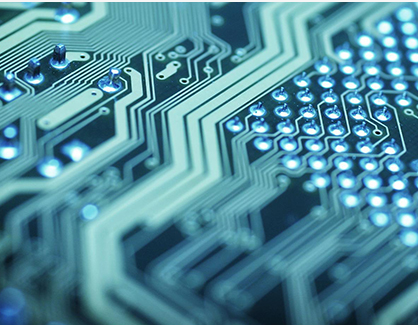
The key to horizontal electroplating is to create a suitable horizontal electroplating system that can enable highly dispersed plating solutions to exhibit superior functional effects compared to vertical electroplating methods through improved power supply and other auxiliary devices.
I, Overview
With the rapid development of microelectronics technology, printed circuit board manufacturing is rapidly developing towards multi-layer, integrated, functional, and integrated directions. The concept and design of circuit graphics using a large number of small holes, narrow spacing, and fine wires in printed circuit design have made the manufacturing technology of printed circuit boards more difficult, especially when the aspect ratio of through holes in multi-layer boards exceeds 5:1 and deep blind holes are widely used in laminated boards, making the conventional vertical electroplating process unable to meet the technical requirements of high-quality and high reliability interconnection holes. The main reason for this is to analyze the current distribution status based on the electroplating principle. During actual electroplating, it was found that the distribution of current in the hole showed a waist drum shape, causing the current distribution in the hole to gradually decrease from the hole edge to the center, resulting in a large amount of copper deposition on the surface and hole edge. It is not possible to ensure the standard thickness of the copper layer in the center of the hole that needs copper. Sometimes, the copper layer is extremely thin or there is no copper layer, which can cause irreversible losses in severe cases, Causing a large number of multi-layer boards to be scrapped.
To address product quality issues in mass production, current and additive solutions are currently being used to address deep hole electroplating issues. In the copper electroplating process of high aspect ratio printed circuit boards, most of it is carried out under relatively low current density conditions with the assistance of high-quality additives, moderate air stirring, and cathode movement. By increasing the electrode reaction control area in the hole, the effect of electroplating additives can be displayed. In addition, the cathode movement is very conducive to improving the deep plating ability of the plating solution, increasing the polarization of the plating piece, and compensating for the formation rate of crystal nuclei and grain growth rate during the electroplating crystallization process of the coating, thus obtaining a high toughness copper layer.
However, when the aspect ratio of the through-hole continues to increase or deep blind holes appear, these two process measures become ineffective, leading to the emergence of horizontal electroplating technology. It is a continuation of the development of vertical electroplating technology, which is a novel electroplating technology developed on the basis of vertical electroplating process. The key to this technology is to create a suitable and mutually supporting horizontal electroplating system, which can enable the plating solution with high dispersion ability to display more excellent functional effects than the vertical electroplating method with improved power supply and other auxiliary devices.
II, Introduction to the Principles of Horizontal Electroplating
The method and principle of horizontal electroplating and vertical electroplating are the same, both of which must have both positive and negative electrodes. After being electrified, an electrode reaction is generated to ionize the main components of the electrolyte, causing the charged positive ions to move towards the negative phase of the electrode reaction zone; The charged negative ions move towards the positive phase of the electrode reaction zone, resulting in the deposition of metal coatings and the release of gas. Because the process of metal deposition at the cathode is divided into three steps: diffusion of metal hydration ions towards the cathode; The second step is for metal hydrated ions to gradually dehydrate and adsorb on the surface of the cathode as they pass through the double layer; The third step is for the metal ions adsorbed on the cathode surface to receive electrons and enter the metal lattice.
From actual observation, the situation of the work tank is an unobservable heterogeneous electron transfer reaction between the interface between the solid electrode and the liquid electroplating solution. Its structure can be explained by the double layer principle in electroplating theory. When the electrode is a cathode and in a polarized state, cations surrounded by water molecules and carrying positive charges are arranged in an orderly manner near the cathode due to electrostatic forces. The phase plane formed by the center point of the cations closest to the cathode is called the Helmholtz outer layer, which is about 1-10 nanometers away from the electrode.
However, due to the total amount of positive charge carried by the cations in the outer layer of Helmholtz, its positive charge is not sufficient to neutralize the negative charge on the cathode. The plating solution far from the cathode is affected by convection, and the concentration of cations in the solution layer is higher than that of anions. Due to the smaller electrostatic force acting on this layer compared to the Helmholtz outer layer and the influence of thermal motion, the cation arrangement is not as tight and neat as the Helmholtz outer layer, and this layer is called the diffusion layer. The thickness of the diffusion layer is inversely proportional to the flow rate of the plating solution. That is to say, the faster the flow rate of the plating solution, the thinner the diffusion layer, and the thicker the reverse. Generally, the thickness of the diffusion layer is about 5-50 microns. The distance from the cathode is further, and the plating layer reached by convection is called the main plating solution. Because the convection generated by the solution can affect the uniformity of the plating solution concentrati







 Nov. 05, 2021
Nov. 05, 2021